1. Schematic and working principle of biochemical testing machine
Biochemistry testing machines from simple to modern are based on the principle of colorimetric method. The solution to be measured is transferred into the cell. A light source with white light passes through the filter to obtain a wavelength that is suitable for the solution to be measured, the optical detector captures the intensity of light passing through the cell containing the solution to be converted into an electrical signal, from the signal. This signal machine can calculate and display results.
Schematic diagram of a biochemical testing machine is presented as follows:
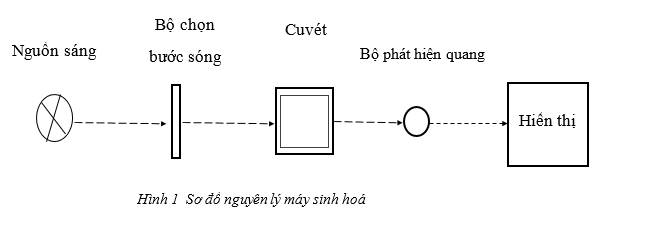
1.1. Light source
The light source is responsible for emitting strong enough white light. The reason for using the white light source here as the biochemical basis we know, is that each test reaction will give a specific color of that test, and it will absorb the strongest range of wavelengths. respectively, so when measuring absorption we use only one basic wavelength. For many tests we will use different wavelengths and the white light source will provide these wavelengths for all tests.
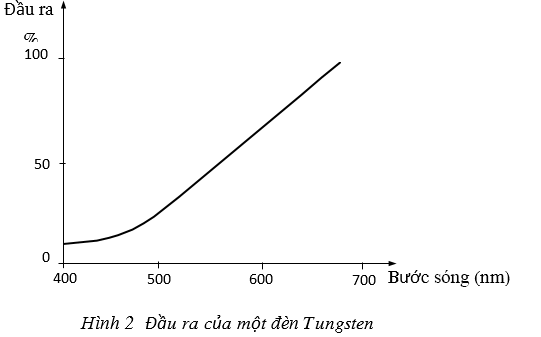
In fact one can easily create a white light source. Figure 2 illustrates the output of a tungsten lamp. We see that the energy decreases towards the ultraviolet but it is still strong enough to power the optical detector at a wavelength of nearly 350nm. To increase the light intensity to the ultraviolet range, a tungsten halide lamp is used. This lamp consists of a tungsten filament placed in a quartz case containing halogen like iodine.
The mercury lamp is a typical example of a discharge lamp across the gas layer, which produces strong emission in the blue spectrum and the ultraviolet band.
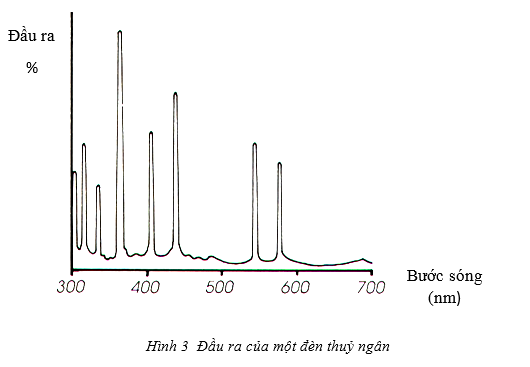
The main disadvantage of mercury lamp is that it can only be used at specific wavelengths. To overcome this drawback, a deuterium lamp is used, despite its high cost and relatively short lifespan. Deuterium lamps emit light of wavelengths up to 190nm, the wavelength range is suitable for most tests today.
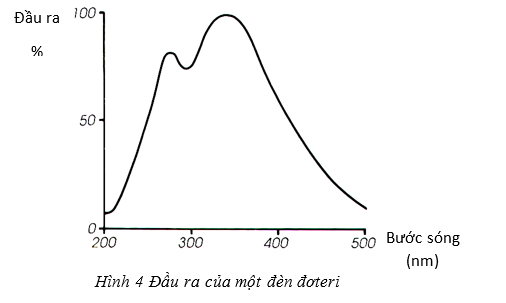 \
\
1.2. Wavelength filter
The wavelength filter is used to select the required wavelength for each test. The reason people use white light sources and filters without using components that emit fixed wavelengths is because using filters can easily add filters according to test requirements, which is open to more testing than using a device that emits a fixed wavelength. In current biochemical testing machines, the filter is usually an upper wheel with a number of filters attached, the number of filters on this wheel depends on the type of machine. These filters are grating, filters, prisms combined with lenses to obtain a very narrow range of wavelengths: 340nm, 405nm, 505nm, 546nm, 570nm, 600nm, 650nm, 700nm, etc.
1.3. Optical detector
Optical detector has the function of converting the optical signal obtained when light passes through the cell into an electrical signal. Optical detector is one of the photo-electric components considered in the previous section, in fact, it is often used as photo diode or transistor photo due to its small size, suitable for laptops or structural machines. small. At the same time, there is a higher sensitivity than other components.
1.4. Display
Measurement results will be displayed on the display block. Depending on the type of machine, there are different ways to display the results, it can be simply displayed as a digital on the 7-bar led, displayed on the CRT screen or displayed on the liquid crystal display (LCD) . From there, the displayed results can be as simple as the amperage obtained, or calculated to display details to the absorbance, substance concentration, patient name, ordinal number, date of review. experience ...
2. Methods of measurement in biochemical tests.
2.1. Endpoint method
This method only measures the absorbance of the reaction mixture once, at which point the coloring reaction has completely formed, and the concentration of the substance in the solution after the reaction has stabilized. Absorbance can be measured at one wavelength (monochromatic) or two wavelengths (bichromatic). Use the standard solution (Standard) to build the calibration curve or preset factor.
Divide by the number of standard solutions used when measuring we have 3 types:
- Measurements using only one standard solution: the concentration of the sample is also calculated by the formula:
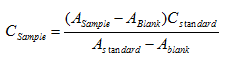
Measurements using multiple standard solutions: the concentration of the sample is determined from the calibration curve. This curve is constructed from known standard solutions of measured concentration and absorbance:
(Astandard– Ablank) .TR
TR is the direction of reaction: +1 if the reaction rate increases
-1 if the reaction rate decreases
The concentration of the sample is interpolated from the standard curve: (Asample - Ablank) .TR
- Measurements using coefficients:
When measuring, do not use standard solutions but use a given factor to calculate the concentration of the solution. The sample concentration is calculated by the formula:
Csample = (Asample– Ablank) .F
Where F is a given factor
Dividing by the number of wavelengths used when measuring we have 2 types:
- Single wavelength measurement: measure the absorbance of the White, Standard and Sample solutions at one wavelength.
- Two wavelength measurement: measure the absorbance of White (Blank), Standard and Sample (Sample) at two wavelengths, one main wavelength and one secondary wavelength, the absorbance of the solution is calculated by the formula after:
Asample, Astandard, Ablank = Almain– Alref
A: absorbance
lmain: Main wavelength
lref: Secondary wavelength
2.2. Kinetic method (Kinetic)
Kinetic methods are used to measure enzyme activity. The characteristic of this method is to measure at the beginning of the reaction, after a delay (delay), there is no stable waiting time.
- Dynamic measurement method (K):
The absorbance of the reaction mixture is measured n times in reaction time t (Reaction time), the distance between n measurements is t / n seconds and the difference value of absorbances between the following measurements is Previous measurement, the final result is the calculation of the average difference value / minute (DA / min)
The activity of an enzyme is calculated by the formula:
Enzyme activity = DA / min.K.
In which, K is the coefficient usually given for each chemical.
The unit of measurement of enzyme activity is U / L, in addition to a new unit, kat (katal), is the amount of yeast that catalyzes 1 mol of substrate in 1 second.
- Fixed time measurement method (Fixed Time):
Similar to kinematic measurements, but the only time between absorbance measurements is fixed.
Hotline